Restorative Therapies Around the World:
 Argentina
Australia
The Baltics
Brazil
Canada
Chile
Czech Republic
Denmark
Germany
Hong Kong
India
Ireland
Italy
Malaysia
New Zealand
Paraguay
Poland
Russia
Saudi Arabia
Singapore
Thailand
Turkey
United Arab Emirates
United Kingdom
Uruguay
Vietnam
Argentina
Australia
The Baltics
Brazil
Canada
Chile
Czech Republic
Denmark
Germany
Hong Kong
India
Ireland
Italy
Malaysia
New Zealand
Paraguay
Poland
Russia
Saudi Arabia
Singapore
Thailand
Turkey
United Arab Emirates
United Kingdom
Uruguay
Vietnam
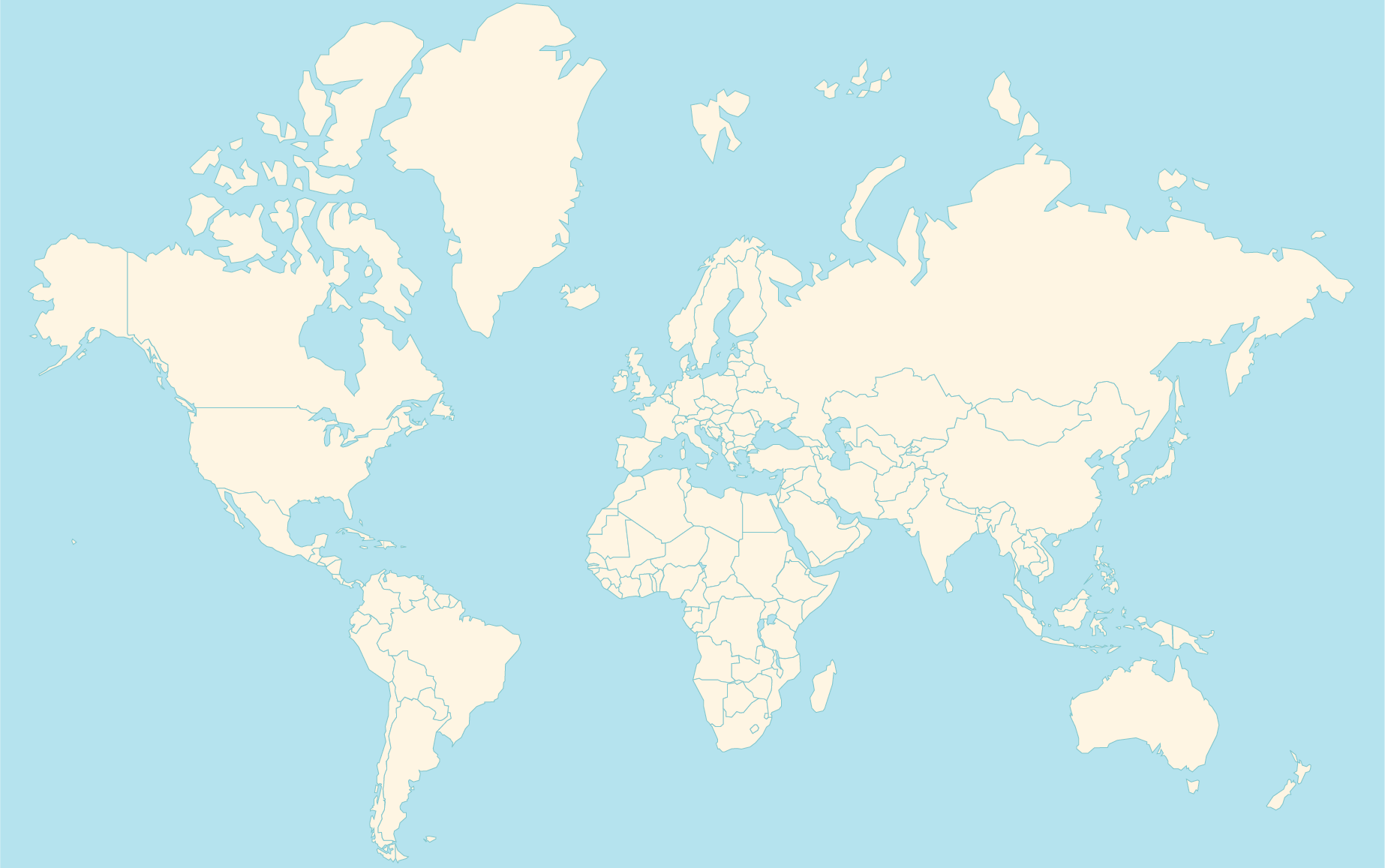
Choose a Distributor:
To locate a distributor, please click the marker on the map for your area. If your country is not noted, please contact support@restorative-therapies.com

Argentina

The Baltics

Brazil

Canada

Chile

Czech Republic

Denmark

Germany

Hong Kong

India

Italy

Malaysia

New Zealand

Paraguay

Poland

Russia, Armenia, Azerbaijan, Belarus, Kazakhstan, wKyrgyzstan, Moldova, Tajikistan, Uzbekistan, Georgia, Ukraine

Saudi Arabia

Singapore

Thailand

Turkey

United Arab Emirates

Uruguay

Vietnam
Direct Sales
International Regulatory Information
Certifications
CE production Quality Assurance
Directive 93/42/EEC
Quality Management System
ISO13485:2016; EN ISO 13485:2016
MDSAP (ISO13485:2016)
Restorative Therapies, Inc is audited every year by:
SGS USA
75 Passaic Avenue
Fairfield, New Jersey 07004
United States



United States
FDA 510(k) cleared devices:
RT300: K060032 K050036 K071113 K071486 K072398 K090750 K162470
RT600: K103366
RT200: K103370
Xcite: K160614
FDA listing number:
RT300: D303875
RT600: D198138
RT200: D203325
Xcite: D284299
FDA registration number: 3004733458
Primary SIC: 3841 (Surgical And Medical Instruments)
Primary NAICS: 334510 (Electromedical and Electrotherapeutic Apparatus Manufacturing)
Nationally Recognized Testing Laboratory (NRTL) Listing:
RT300-SL, SLSA, SLP, LSA, SLA
XciteV2
Maryland Residential Service Agency license:
R3932
Argentina
Declaration of Conformity:
RT300, RT200, RT600, Xcite
Australia
Business registration:
ABN 28 131 719 661
ARTG Certificates:
RT300, RT600, RT200: ARTG# 176355
Xcite: ARTG# 284186
Declaration of Conformity:
RT300
RT600
RT200
Xcite Clinical Station
Sponsor for regulatory affairs issues and incident reporting:
Emergo Australia
Level 20, Tower II
Darling Park
201 Sussex Street
Sydney, NSW 2000
Australia
Canada
Medical device licenses:
RT300
RT600
RT200
Xcite Clinical Station
RT60 six channel stimulator
RT50 single channel stimulator
Canadian Standards Association (CSA) Listing:
RT300-SL, SLSA, SLP, LSA, SLA
RT300 Supine/SA
RT600
Xcite

Europe
Declaration of Conformity:
RT300
RT600
Xcite Clinical Station
Representative for regulatory affairs issues and incident reporting:
Emergo Europe
Prinsessegracht 20
2514 AP The Hague
NETHERLANDS
Tel: +31 (0) 70 345 8570

Hong Kong
Declaration of Conformity:
RT300

India
Authorized Representative
NV Genomics Pvt Ltd
95 Sector 19, Pocket 3,
Dwarka, New Delhi, Delhi 110075, India
https://www.nvgenomics.com/
Kazakhstan
Regulatory Approval:
RT300, RT600, RT200
Malaysia
Regulatory Approval:
RT300
Xcite Clinical Station
New Zealand
WAND Registration:
RT300
Xcite Clinical Station

Russia
Regulatory Approval:
RT300, RT600, RT200


Turkey
Regulatory Approval:
RT300
United Arab Emirates
Regulatory Clearance:
RT300 (DRCLAS-2021-005092)
Xcite (DRCLAS-2021-005094)